JACKSONVILLE, Fla. — The first COVID-19 vaccine shots have been put into the arms of our youngest children.
Moderna announcing Tuesday it’s in phase 2/3 of pediatric clinic trials, which includes children in the U.S. and Canada between the ages of 6 months and 11 years old.
UF Health Jacksonville Chief of Pediatric Infectious Diseases and Immunology Dr. Mobeen Rathore said KidCOVE is the first large COVID-19 vaccine study of children, which will enroll about 6,750 kids.
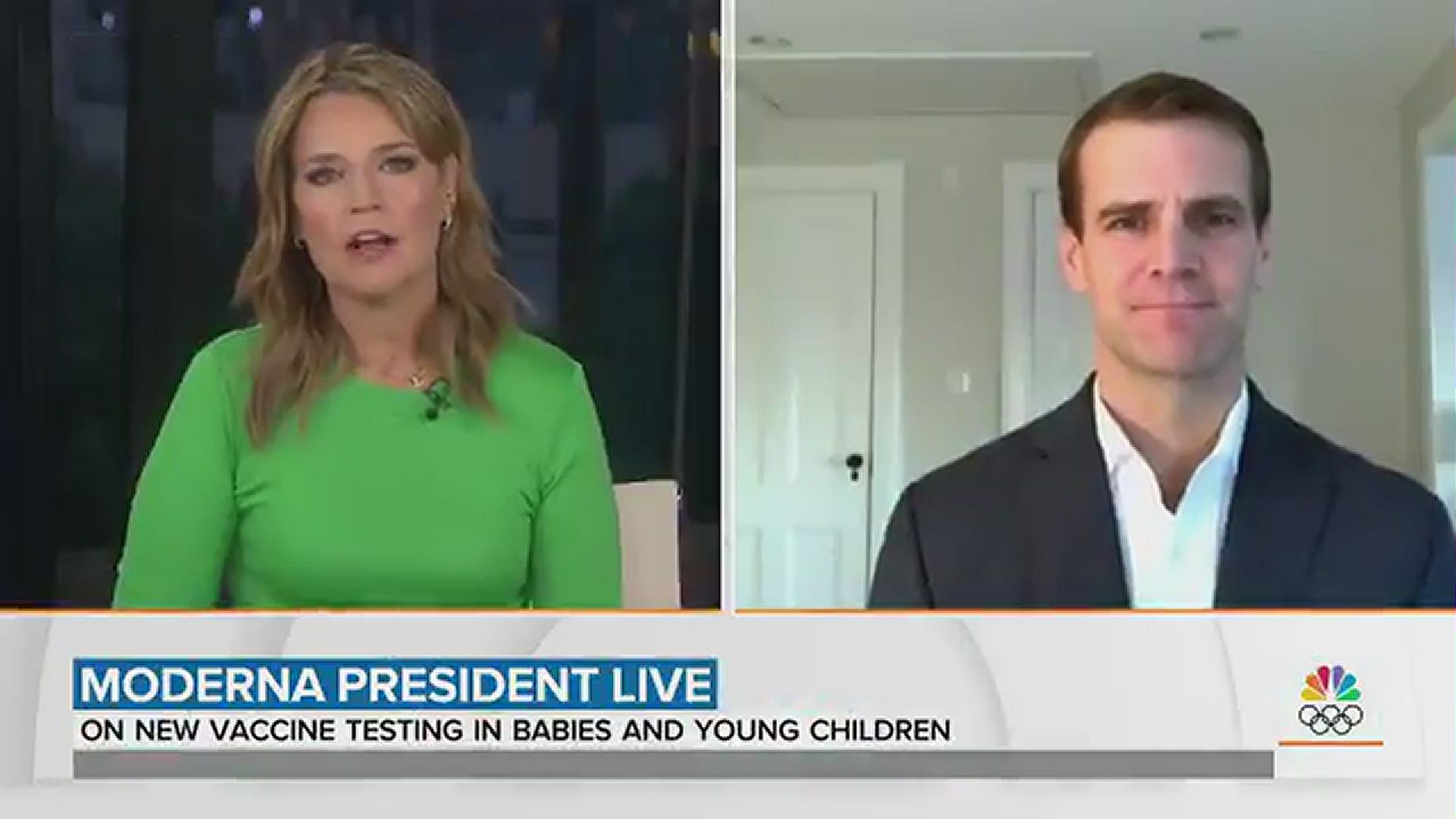
In an exclusive interview with the TODAY show, Savannah Guthrie spoke with Stephen Hoge, President of Moderna.
"How quickly do you expect results from those trials, and can we start seeing kids get vaccinated before the school year next fall?" Guthrie asked.
"It depends on ages," said Hoge, who suggested that children could be vaccinated going into the 2021 school year in the fall.
Here on the First Coast, Rathore said UF Health Jacksonville will be participating and will recruit about 75-100 healthy, local children.
“It's a very important element in our fight against COVID-19, because we're not going to get to herd immunity until we immunize kids," he explained.
However, almost every mother on the First Coast News Facebook page wrote things such as the following:
“Haha not a chance! Protect your babies at all costs!"
“My kids are not science projects. Nope”
But, Dr. Rathore said they shouldn’t be concerned.
“We have to talk to the parents to discuss it with them. And hopefully, we will be able to convince them," he added. "But, this is pretty normal in vaccine research in children where we give children unapproved drugs with all the precautions and the safety measures.“
Back in December, First Coast News began following 14-year-old Meredith Anglin who took part in a local Pfizer COVID-19 vaccine trial.
“For some reason, the second shot hurt worse than the first one. But it still wasn't very bad at all," Anglin explained. "Personally, I didn't experience any side effects.“
She’s still waiting on the results of her adolescent study to be unblinded, but encourages our youngest to play an important role in seeing how the vaccine affects children.
“I would love to help the research," she said. "And I want everybody to be able to get vaccinated as soon as possible so we can get this [pandemic] over with.“
The Pfizer COVID-19 vaccine is being studied in children as well.
Johnson & Johnson has also announced plans to study the vaccine in adolescents, ages 12 to 18.